New ‘Cysteine Fingerprinting’ Technique Revolutionizes Protein Identification
Identifying Proteins in a Blink of an Eye: Technion investigators develop a novel method using DNA tags and machine learning to slow, capture, and rapidly identify single, full-length proteins with near-perfect accuracy.
A team of investigators led by Prof. Amit Meller from the Technion, including the postdoctoral fellows Neeraj Soni, Navneet Verma, the graduate students Zohar Rosenstock and Noam Talor and the team scientists Barak Marom, in collaboration with investigators from the University of Illinois at Urbana–Champaign and Rice University, Houston developed a groundbreaking method to identify and classify individual, full-length proteins by creating a unique "fingerprint" based on their cysteine (a particular amino acid) content. The technique, which uses solid-state nanopores, solves two of the biggest challenges in single-molecule protein analysis: it dramatically slows the protein's rapid movement through the sensor and increases its capture rate by a factor of ten.
The new approach, detailed in a recent publication in Nature Nanotechnology, offers a rapid, direct, and cost-effective platform for protein identification that eliminates the need for expensive and highly specific reagents like antibodies.
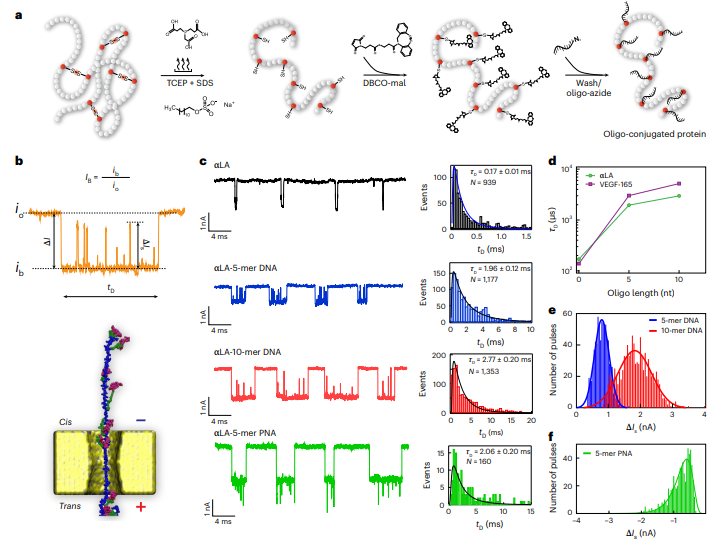
Analyzing single proteins with nanopores—tiny, nanoscale holes that measure electrical changes—has historically been difficult because the proteins translocate, or pass through, too quickly to be read accurately. To solve this, the research team devised a novel strategy: using "click chemistry" to attach short, negatively charged DNA strands (oligonucleotides) to cysteine residues, a common amino acid.
These DNA tags serve two critical functions (1) Increased capture: The strong negative charge of the DNA tags acts as an "electric handle," making it over ten times more likely that a protein will be captured from a solution and threaded into the nanopore, and (2) Slower translocation: The tags temporarily interact with the nanopore's surface, creating a "stick–slip" motion. This novel mechanism, confirmed by all-atom molecular dynamics simulations, slows the protein's journey by more than 20-fold, giving the sensor ample time to read it.
As each DNA-tagged cysteine on the linearized protein passes through the pore, it generates a distinct, positive pulse in the ion current. The resulting sequence of electrical pulses acts as a unique, time-resolved "fingerprint" corresponding to the spatial arrangement of cysteines along the protein's chain.
"Our approach transforms the challenge of rapid translocation into a feature," said Prof. Amit Meller, the lead author on the study. "By tagging the proteins, we not only slow them down but also generate a high-fidelity electronic signature that machine learning (ML) can easily decipher. This opens the door to a new kind of protein analysis that is direct, rapid, and doesn't rely on antibodies."
The team fed these unique electrical fingerprints into a supervised machine learning classifier. The model was able to identify individual proteins from a complex mixture with near-perfect classification accuracy using just a small number of translocation events.
To demonstrate the method's powerful diagnostic potential, the researchers successfully used it to distinguish between two critical cancer-related protein isoforms, VEGF-165 and VEGF-121. These isoforms are notoriously difficult to tell apart with conventional methods but were easily classified using the new nanopore technique, even when mixed together.
This "cysteine fingerprinting" technique offers a robust and cost-effective platform for single-molecule proteomics. Given that 97% of all human proteins contain at least one cysteine residue, the method is broadly applicable to a vast portion of the proteome. The researchers anticipate that future work could expand this chemical tagging strategy to other amino acids, paving the way for a highly sensitive, portable platform for comprehensive single-molecule protein analysis.
For the full article in Nature Nanotechnology